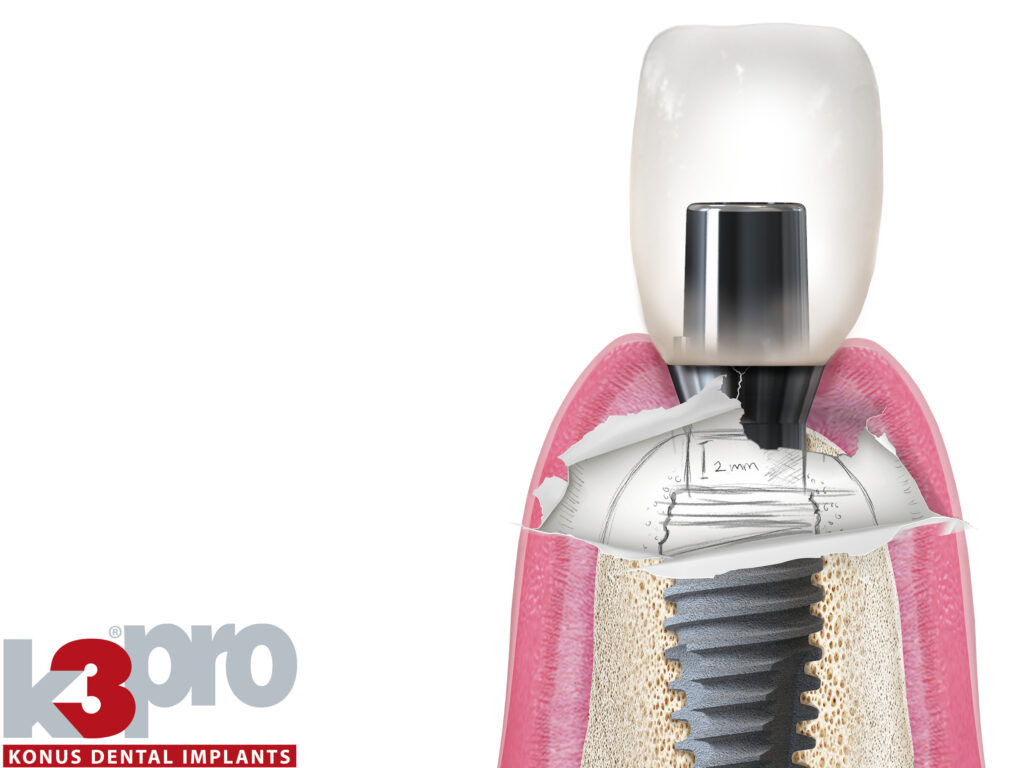
Die bewährte Philosophie von K3Pro® ist der subkrestale Einsatz. Durch den absolut bakteriendichten und mikrobewegungsfreien Konus und die abfallende Schulter heilt das Implantat tief in den Knochen ein. Die Gingiva haftet dauerhaft an.
Durch den Form- und Kraftschluß des 1,5°-Konus erzielen wir das beste beider Welten: die chirurgische und prothetische Flexibilität des zweiteiligen Systems bei gleichzeitiger Lastverteilung und Stabilität wie bei einteiligen Implantaten. Die Abstände zu benachbarten Zähnen oder Implantaten können deutlich reduziert werden.
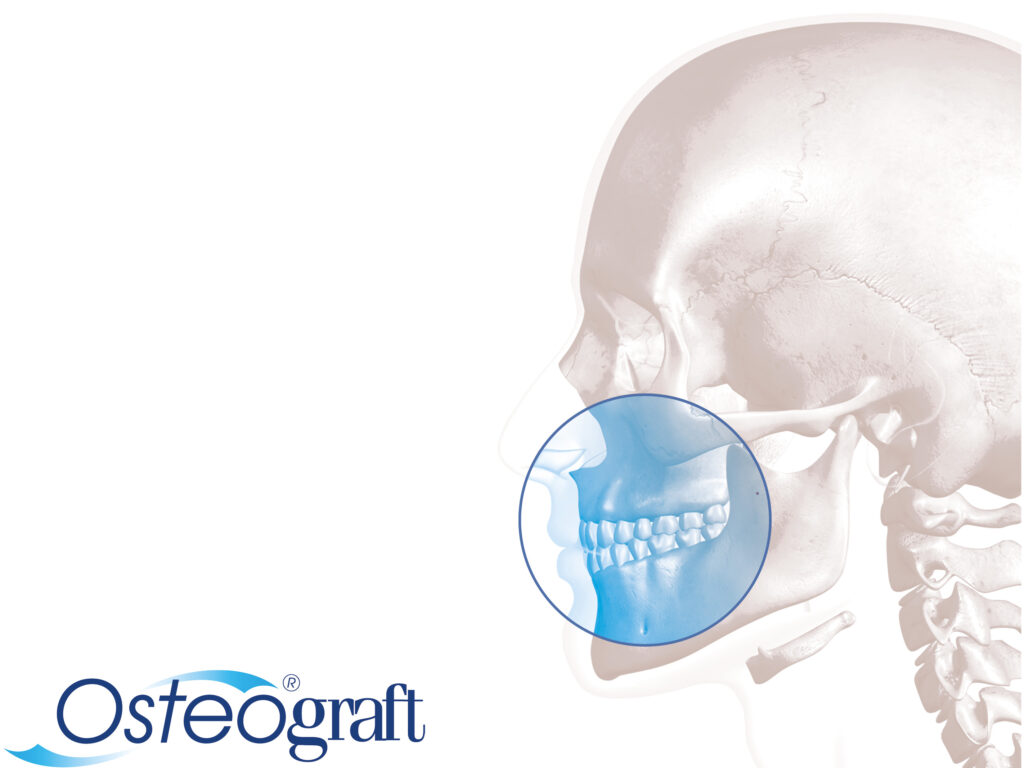
Allogene Transplantate sind ein weiterer Erfolgsfaktor des Unternehmens. Mit Osteograft stellt Argon Produkte zur Verfügung, die allen Kriterien der modernen augmentativen Chirurgie für GTR- und GBR-Anwendungen gerecht werden. Der hohe Anspruch der Unternehmensleitung an maximale Sicherheit wird durch die Zusammenarbeit mit dem Deutschen Institut für Zell- und Gewebeersatz (DIZG) in Berlin erfüllt. Osteograft-Transplantate werden vom DIZG aus dem deutschen Spenderprogramm hergestellt und sind als Arzneimittel zugelassen. Umfangreiche Kriterien zur Spenderauswahl, ein Spenderscreening, das die EU-Richtlinie übererfüllt sowie ein validiertes Sterilisationsverfahren zur Entfernung bzw. Inaktivierung von Viren, Bakterien und Pilzen bilden die Grundlage für größtmögliches Produktvertrauen bei Behandlern und Patienten.
Quickfinder Produkte:
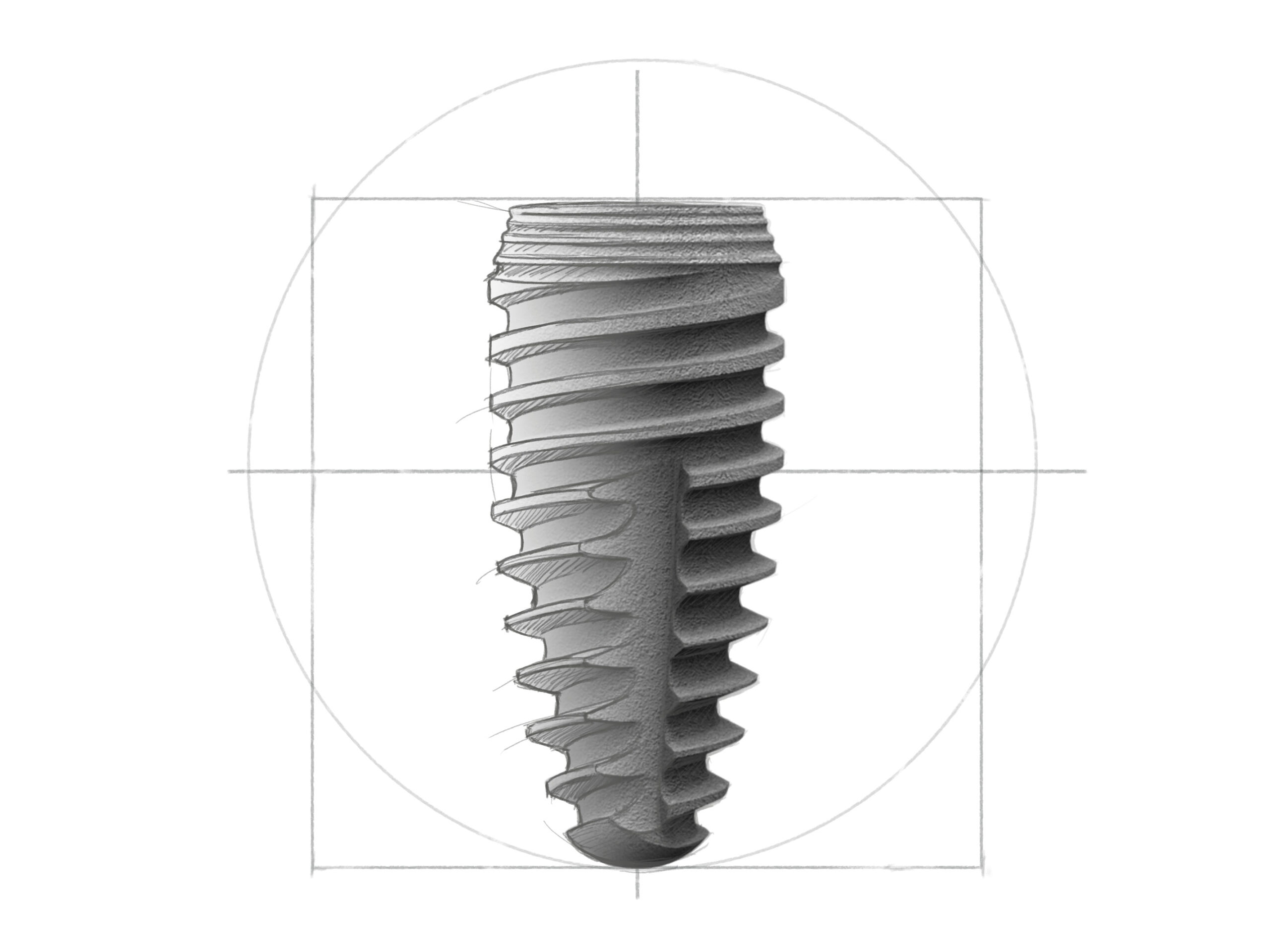
R-Line
Rapid Implantate mit selbst schneidendem Gewinde und konischem Implantatkörper. Es kann nach der Osteotomie noch in der Richtung variiert werden. Schneidgewinde und Schneidnut erlauben eine gesteuerte Platzierung des Implantats.
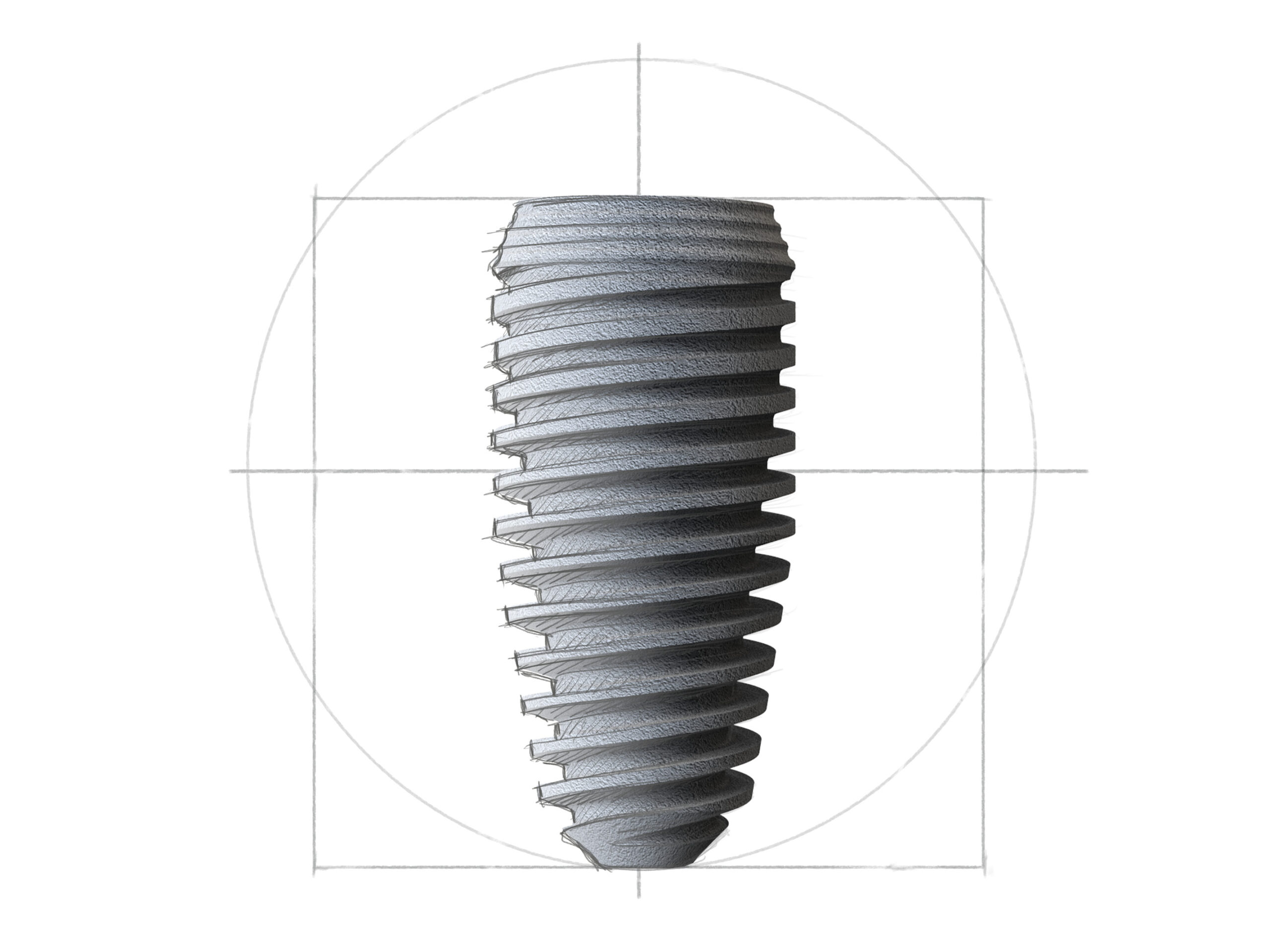
C-Line
Das Design der evolutionären Compress Implantate zeichnet sich durch ein einzigartiges Plateau-Schneidgewinde aus, das insbesondere im weichen Knochen eine besonders zuverlässige hohe Primärstabilität gewährleistet.
S-Line
Sure Implantate mit Kompressions-Gewinde-Design. Die Osteotomie gibt die Richtung der Implantatinsertion vor. Das Parallel-Gewinde sowie der leicht zylindrisch und apikal konisch ausgelegte Implantatkörper bieten eine große Oberfläche.
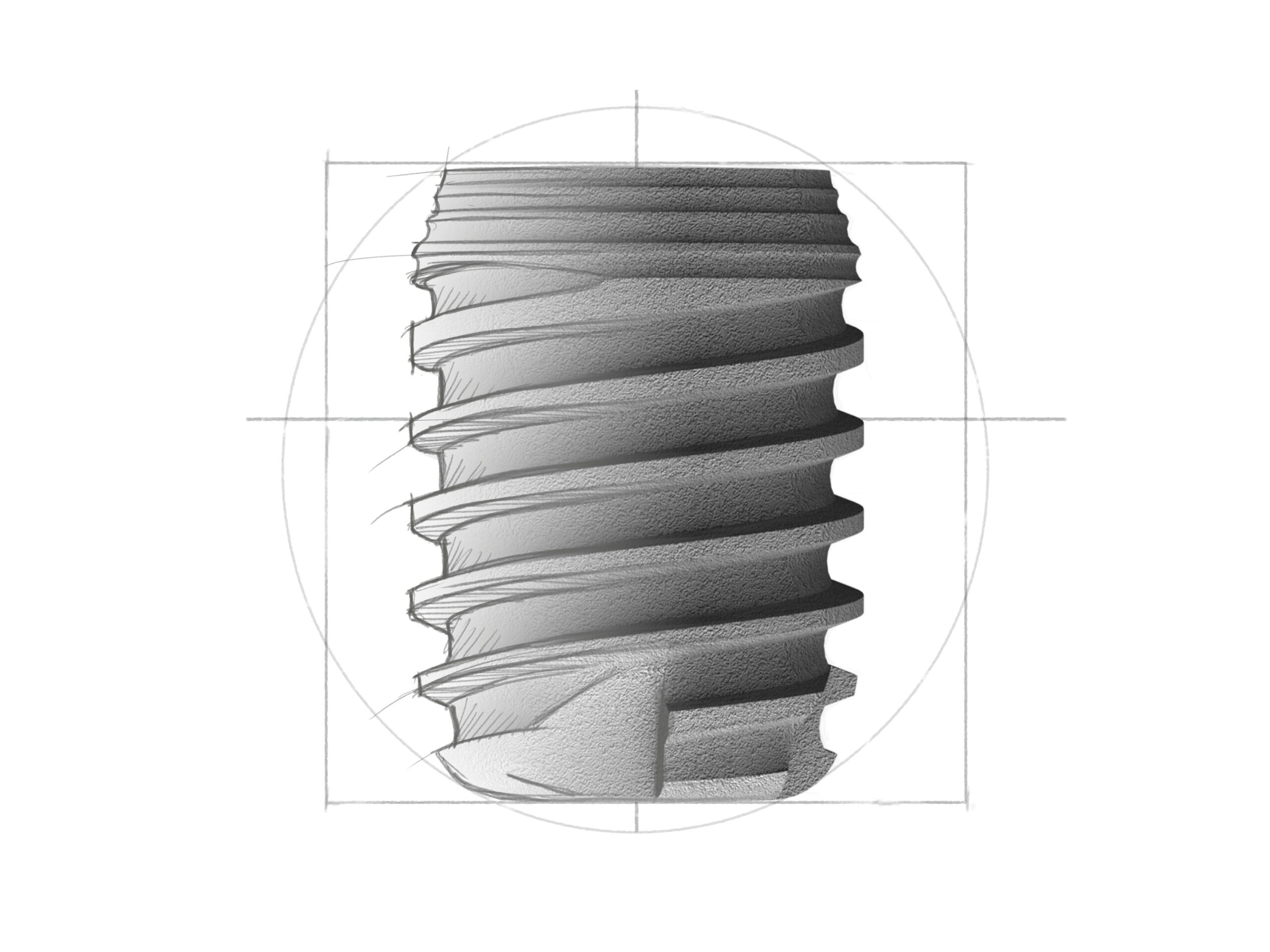
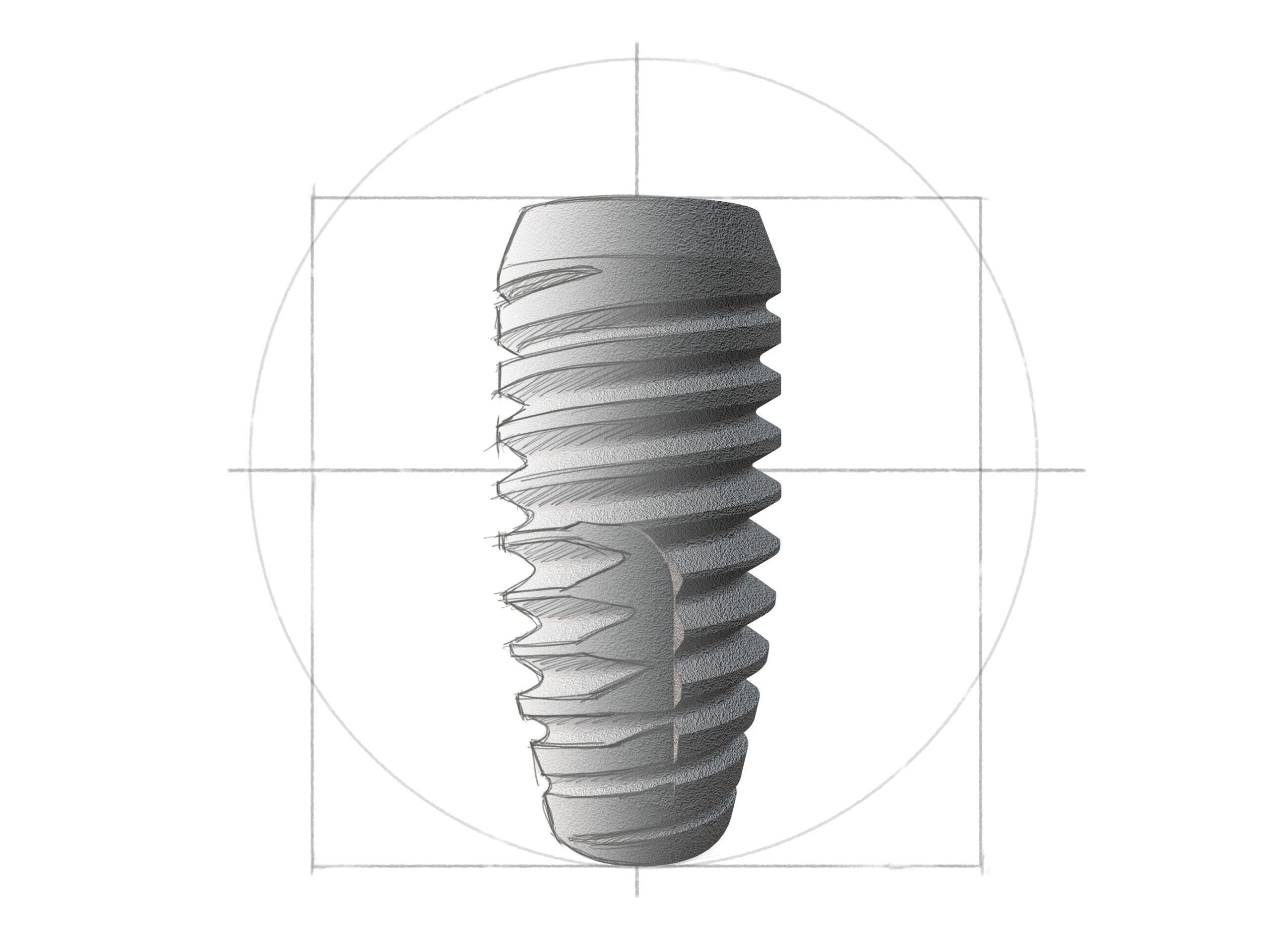
Short
Short Implantate für schwierigste Indikationen bei stark reduziertem Knochenangebot mit der revolutionären, langen Konusverbindung mit 1,5°-Winkel. Dies gewährleistet nachhaltige Bakteriendichtigkeit und Mikrobewegungsfreiheit.

Allogene Granulate
Spongiosa-, Corticalis- und Corticospongiosagranulate bieten hohen Resorptionsschutz für Volumenstabilität. Sie dienen als Basis für Membran Techniken wie Tent-Pole oder Umbrella und sind ideal für die Füllung in Schalentechnik sowie die Konturierung von Knochenblock-Onlays.

Blöcke, Würfel & Späne

DBM pastös
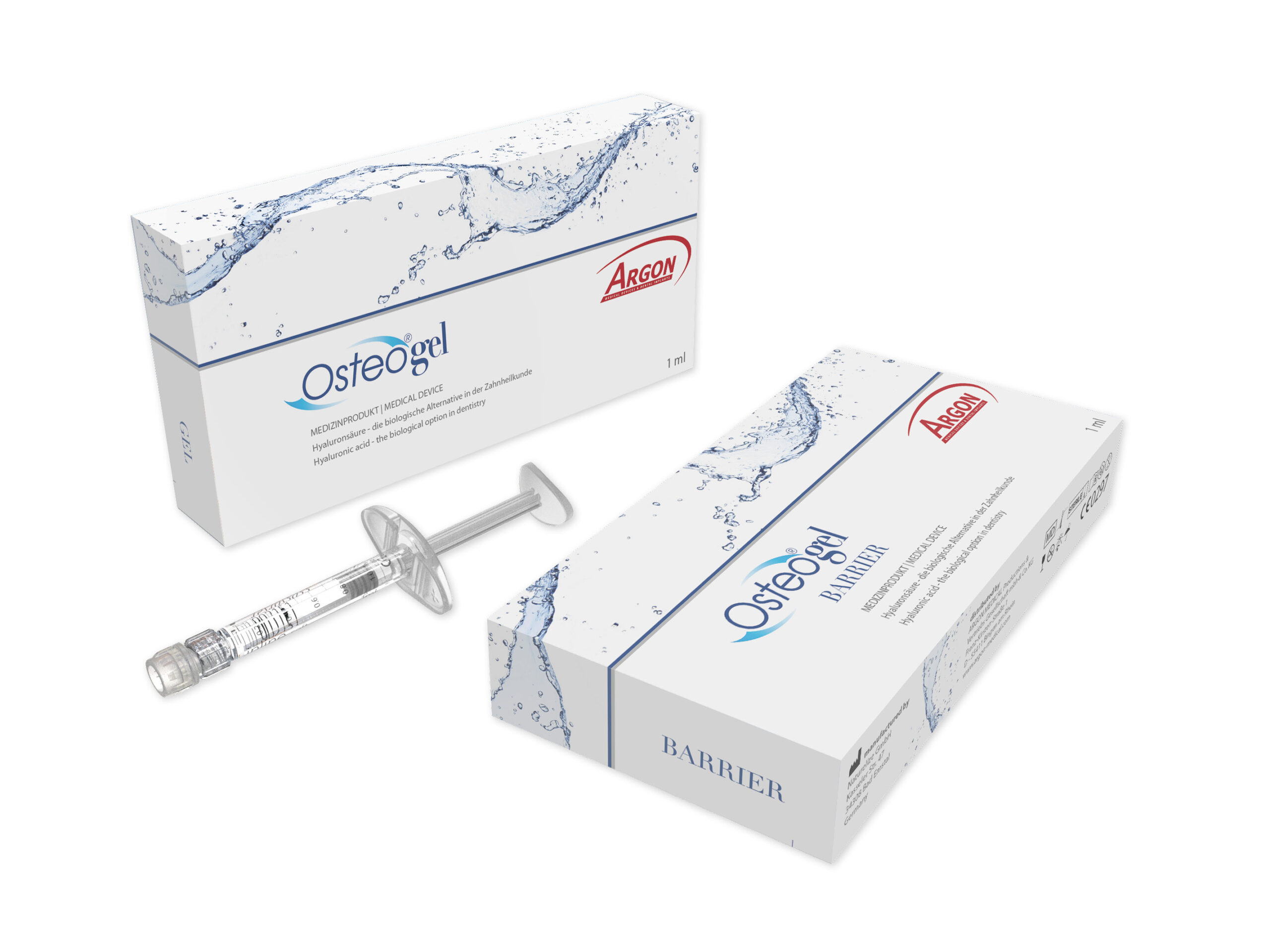
Osteogel/Osteogel Barrier
Unvernetzte Hyaluronsäure speziell zur Behandlung von Entzündungen der Mundschleimhaut und zur verbesserten bzw. gesteuerten Wundheilung nach chirurgischen Eingriffen. Perfekt geeignet als Trägermaterial für Granulate des gesamten OsteoGraft Produktportfolios.
